In laboratories around the world, scientists are racing to build defenses against the tide of plastic pollution. Some efforts focus on the environment itself — filters in rivers, enzymes in landfills, biodegradable packaging that dissolves into harmless compounds. Others look inward, to the body, asking how we might shield and protect ourselves from the fragments already saturating air, food, water and our cells.
Origins in Defense
The roots of Qi601 trace back to the Quorum Innovations™ central concept of microbial biofilms for human body defense. Years ago, Quorum Innovations founders had what they believed was a seminal realization: that the human microbiome itself lives on the body’s surfaces as a surface-protective barrier — as a protective biofilm. This assumption — that microbes are not only inhabitants but guardians of epithelial surfaces — has guided Quorum Innovations’ design of probiotic-inspired biofilm shields. Quorum Innovations received two Defense Advanced Research Projects Agency (DARPA) awards to further develop its human barrier defense technologies for warfighters — protections against chemical and biological weapons in combat. That work focused on the natural properties of microbial biofilms: sticky, layered communities that resist chemical invasion by trapping or deflecting foreign and chemical particles. In parallel to working with DARPA, Quorum partnered with Bayer Pharmaceuticals in a joint development program exploring biofilm barrier protection technologies, further validating the translational potential of this approach. From defense against battlefield chemicals to pharmaceutical collaborations, the concept of “surface protection” built on protective biofilms is the foundation upon which Quorum’s technology was built. Ultimately, Quorum Innovations would determine that Qi601 could also defend civilians against the invisible invasion of plastics.
Biofilm Shield: How Microbial Communities Become Protective Barriers
A bacterial biofilm is a highly organized, cooperative community of microbes that behaves very differently from individual, free-floating (planktonic) bacterial cells. Within a biofilm, microbes communicate using quorum-sensing molecules that trigger synchronized, population-wide shifts in gene expression, effectively creating a new phenotype with enhanced resilience and shared capabilities. These communities also form electrical channels that allow cells to exchange information and nutrients, functioning almost like a cohesive multicellular organism. Encasing the entire consortium is an extracellular polysaccharide matrix — a sticky, protective scaffold that anchors the biofilm to surfaces, shields it from environmental stress, and creates a physical barrier against chemical or biological intrusion. In this way, biofilms demonstrate a fundamental biological principle and phenomenon that illustrates a core biological truth: when living systems act collectively, when acting together as a quorum, they gain emergent properties far greater than the sum of their individual parts – a principle true for people too.

Qi601 was derived from a beneficial human-associated bacterium discovered by Quorum Innovations. This organism was independently reviewed by the U.S. Food and Drug Administration 22
and granted GRAS (Generally Recognized as Safe) status under GRN 000988, confirming its safety profile and suitability for human exposure. From this source, Quorum developed Qi601™ as a non-living, biofilm-derived protective matrix — a Probiomic® — made to bind and sequester micro- and nanoplastics before they interact with human tissue.
Quorum Innovations has also secured extensive intellectual property protection around Qi601 and its underlying biofilm architecture. The company has over 50 issued patents across the United States, the European Union, China, Japan, Korea, Israel, Thailand, and Australia, with approximately 20 additional patents pending worldwide. The USPTO, the European Patent Office, and patent authorities across multiple continents have recognized the uniqueness of Quorum’s discoveries, issuing patents that protect the bacterium itself, its biofilm-derived cellular mass, its manufacturing methods, and its application as a barrier and plastic decontamination technology. This broad and growing portfolio affirms the novelty, scientific merit, and global relevance of Quorum’s inventions — establishing the company as a leader in biofilm-based protective platforms and anchoring Qi601 as a first-in-class solution for defending human health against environmental plastics.
Among emerging strategies for mitigation of plastic absorption by the human body, one stands apart for its elegance and simplicity: Qi601, a product developed by Quorum Innovations. Unlike biotherapeutics that alter metabolism or complex genetic therapies, Qi601 acts as a physical barrier in the gut — a microscopic biofilm shield designed to intercept plastics before they can touch human tissue. Derived from an inactivated Limosilactobacillus fermentum strain to which the FDA granted Generally Recognized as Safe (GRAS) status, Qi601 forms naturally aggregating biofilm probiotic structures. Qi601 is a ProBiomic® functioning as both a defensive surface coating and an intelligent binding matrix that promotes the integrity of the human microbiome. Beyond its barrier role, laboratory experimental findings suggest Qi601 may also protect human cells after plastics have entered the cell, acting as a transepithelial binding agent that establishes a high-affinity extracellular sink for nanoplastic particles. This mechanism may facilitate active transport or exocytic release of internalized plastics from the cell, reducing intracellular burden while preventing further absorption from the cell surface. In this way, Qi601 represents a new class of microbiome-inspired health products — one that not only prevents exposure to plastic absorption but may also help reverse it, transforming the gut epithelium from a passive boundary into an active, absorptive surface. This discovery represents a paradigm shift: not only can Qi601act as a barrier to prevent the absorption of plastics but it also functions as a plastic decontamination agent, facilitating the clearance of plastics that may have already entered cells. Together, these findings suggest that barrier-based protection and intracellular plastic decontamination can coexist within the same biologically inspired technology of Qi601 — offering a new frontier for defending the human body against the invisible burden of micro- and nanoplastics.
The promise of Qi601 is visible, not abstract. In Quorum Innovations’ research labs, initial experiments using fluorescence microscopy demonstrate the preferential and selective adhesion of fluorescent plastic particles to Qi601 aggregates. When exposed to 10-micron green, fluorescent microspheres, the large spherical plastic particles are seen clustered along the irregular surfaces of Qi601, forming bright rings of fluorescence that delineate the Qi601’s biofilm’s contours. (figure 1). In parallel fluorescent microscopy experiments using 100- nanometer fluorescent nanoplastic spheres, the same pattern emerges, this time at the nanoscale: Qi601 aggregates act as discrete focal points of binding of 100-nanometer, fluorescent particles, dusting the surface of Qi601 biofilm aggregates. Areas of the field devoid of Qi601 remain entirely free of fluorescence (figure 2). This staining pattern of both 10 micron and 100-nanometer fluorescent plastic particles confirms that Qi601 exhibits a high-affinity, size-independent attraction for plastic materials, driven by its surface topology and electrostatic properties. The absence of bright green fluorescent particles outside the Qi601 boundaries underscores the selectivity of this interaction and provides direct visual evidence of Qi601’s physical sequestration mechanism, in which plastics of varying sizes adhere only where the Qi601 biofilm shield aggregates are present. Together, these findings provide visual confirmation that Qi601 functions as a physical sequestration platform, capable of attracting and retaining both micro- and nanoplastic contaminants through surface- driven adhesion.

Figure 1: Fluorescence microscopy of Qi601 aggregates bound to microplastics.
Qi601 aggregates approximately 200 µm diameter (shaded area under the “hand”) are coated with multiple, green multiple fluorescently labeled 10 µm polystyrene spheres, demonstrating selective sequestration of plastic particles to Qi601 surface.

Figure 2: Fluorescence microscopy of Qi601 aggregates bound to nanoplastics. Qi601 aggregates with 100 nm green fluorescent nanoparticles, coating the surface selectively and with sensitivity and specificity, confirming high binding affinity of Qi601 and nanoplastics at the nanoscale.
Initial fluorescence microscopy experiments on human colonic epithelial monolayers (the Caco 2 cell line), researchers applied nano sized, green fluorescent plastic particles and watched the cells light up. In untreated control conditions, fluorescent green plastic particles—ranging from 10 micron microplastics to 100-nanometer nanoplastics—formed a dense coating of plastic particles across the epithelial surface of the human colon cells, appearing as a bright green film indicative of strong plastic-human cell interaction (Fig. 3, top panels). Fluorescence microscopy of the human colonic epithelium illustrates the protective binding behavior of Qi601 against micro- and nanoplastic adhesion. (Fig. 3 bottom panels). When Qi601 was introduced into the same experimental system, the distribution of plastic fluorescence changed dramatically (Fig. 3,right upper to right lower panels). Plastics no longer adhered to human colon cells; instead, they aggregated selectively along Qi601 biofilm structures, forming clusters distinct from the epithelial surface. This redistribution of nanoplastic nanoparticles strongly suggests Qi601’s role as a physical barrier, competitively binding nanoplastics particles to divert plastics away from human cellular contact and effectively shielding intestinal lining from direct exposure(Fig. 3).
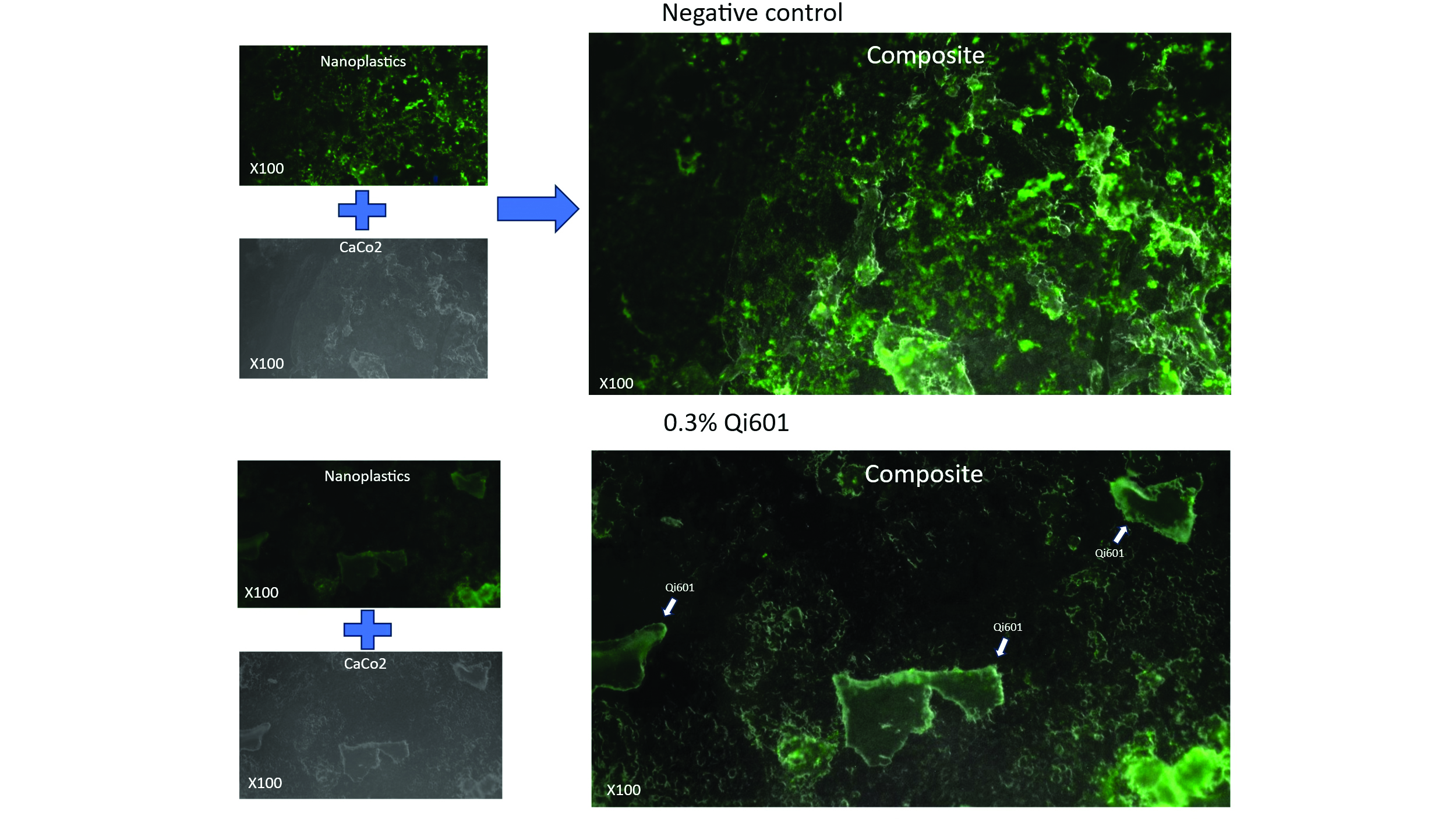
Figure 3: Fluorescence microscopy of nanoplastic binding with and without Qi601. Qi601 diverts
nanoplastics away from human colon epithelial cells, capturing them on its biofilm surface to prevent cellular binding.: Composite fluorescence and bright-field microscopy images of human colon epithelial cells incubated with green fluorescent polystyrene nanoplastic particles are seen above Top panels (negative control) indicate that without Qi601, nanoplastics (green color) adhere extensively to the surface of the human intestinal cells, forming a continuous fluorescent layer blanketed across the cells. Bottom panels (with Qi601) show that when Qi601 is introduced, fluorescent particles are redirected to and clustered upon the Qi601 aggregates (indicated by arrows), leaving the surrounding cells largely free of bound plastics. This shift demonstrates Qi601’s high-affinity sequestration mechanism—capturing micro- and nanoplastic particles before they can attach to epithelial cells and thereby
functioning as a protective biofilm shield at the human gut interface.
Quantitative analysis mirrored what the eye could see—nanoplastic attachment to human colon cells fell by 83% with Qi601 when compared with untreated controls—evidence of a physical and functional barrier operating at the cell interface. These particular results show that Qi601, as a biofilm-inactivated bacterial aggregate, works as a protective shield inside the gut, on human colon cells, capturing plastics before they can attach to human intestinal cells. In biological terms, it creates a physical “sink,” a competitive binding surface, capturing plastic contaminants away from living tissue and onto itself, thereby preventing attachment and absorption across the gut.
A simple bench-top visualization makes the effect visceral. In test-tube fluorescence assays, nanoplastic suspensions glow brightly in the suspension when Qi601 is absent. (Fig. 4, right test tube) With the addition of Qi601, the bright green nanoplastic are bound to Qi601 and leave a clear liquid on top. Plastic particles are drawn down by the weight of Qi601 and incorporate into the Qi601 biofilm aggregates at the bottom of the test tube. (Fig. 4., left test tube). These side-by-side tubes translate microscopy images into an immediate, intuitive visualization of the “binding” of the nanoplastic particles. This visual demonstration shows how Qi601 literally removes plastic contamination from its surroundings.
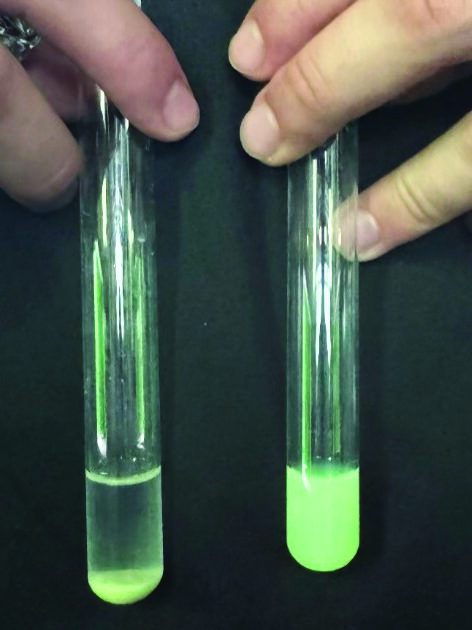
Figure 4: Test tube fluorescent nanoplastic assay. One hundred nanometer, fluorescent nanoplastic suspensions glow vividly on the right test tube when Qi601 is absent. When Qi601 is added, the green fluorescence particles are captured by Qi601, sink to the bottom of the tube and disappear from the liquid above — a striking image of sequestration in action. (JNM)
Atomic force microscopy (AFM) strengthened the picture with image resolution far below what the human eye can see. With nanometer-scale images—on the order of a billionth of a meter—AFM revealed these Qi601 bacterial biofilm aggregates as uneven cobblestone (Fig. 5, blue arrows) like structures: a rugged, topology created by self-assembling, inactivated bacteria. (Fig. 5) Within this cobblestone-like terrain, small nanoplastic particles interdigitated on and between Qi601, (Fig. 5, red arrows) tucked into crevices and held fast by the Qi601 biofilm matrix. The images confirm capture of these nanoplastic particles on nanometer scale and offer a direct visualization and mechanistic rationale on the binding capabilities of Qi601 to nano-sized plastic particles. The rough, high-surface-area architecture presents an energetically and sterically favorable binding surface and creates niches that favor nanoplastic sequestration that can out compete plastic binding to human cells. This microscopic structure explains why the material is so effective in our human cell experiments: its textured surface traps particles like a natural sponge, locking them in place and preventing them from attaching and interacting with tissue. If the nanoplastics can’t bind to human cells, then they will not be absorbed into the body. Qi601 is the first line of defense against absorbing nanoplastics across the human intestinal cellular layer.
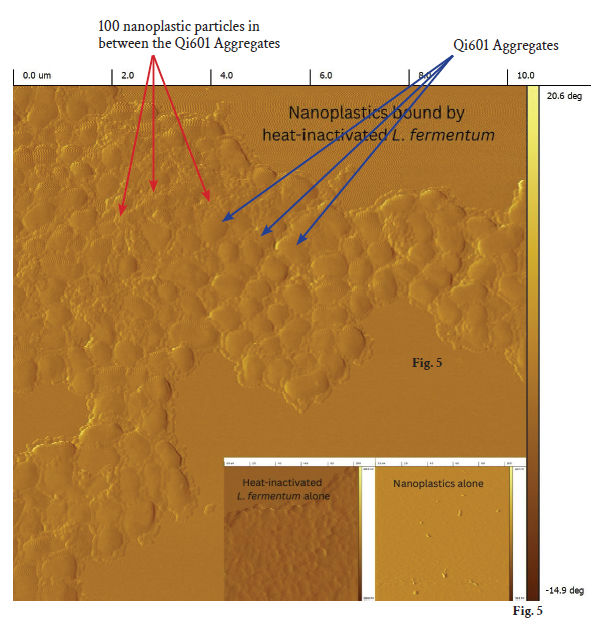
Fig. 5: Atomic Force Microscopy (AFM) of Qi601 Aggregates. AFM imaging of Qi601 aggregates reveals their characteristic cobblestone-like surface topology at the micron and nanometer scales. Each individual aggregate, roughly 150–300 µm in diameter, is composed of densely packed micro-domains formed from the self-assembled aggregates of Qi601 (blue arrows). High-resolution AFM scans show an uneven, biofilm-like matrix with deep interstitial grooves and crevices capable of physically entrapping nanoplastics. When nanoplastics are introduced into the imaging field, they are observed adhering within these depressions and along ridges of the Qi601 surface — a direct visualization of the material’s physical binding behavior (red arrows). This topographic architecture explains the strong sequestration performance seen in fluid assays and cell models: the irregular biofilm terrain dramatically increases available surface area and creates nanoscale pockets of high adhesive potential. These AFM findings confirm that Qi601’s mechanism of action is significantly physical — a biofilm-derived matrix that captures, immobilizes, and retains nanoplastics through texture, topology, and surface chemistry rather than chemical degradation or metabolic interaction. (PNM)
In Quorum Innovations’ laboratory, animal experiments add converging evidence to Qi601 anti-plastic activities. C. elegans is a transparent nematode (worm) widely used in biomedical research for testing gut barrier integrity and inflammation. Animals fed fluorescent nanoplastics showed gut linings that glowed with accumulated, bright green nanoplastic particles (Fig. 6, top panel). By contrast, the intestinal tracts of worms fed Qi601 and nanoplastics exhibited markedly reduced epithelial signal of the fluorescent nanoplastic particles (Fig. 6, bottom panel). The nano sized fluorescence bright green nanoplastic particles present in the nematode gut in the control animals were washed out by Qi601. Excreted nanoplastics ultimately appearing in worm waste products when the worms are simultaneously fed Qi601 (Fig. 6, bottom panel, green arrow). This visual evidence that ingested nanoplastics can be flushed out of the intestinal tract supports the concept that Qi601 not only binds nanoplastics in gastric environment but also flushes the plastic particles out naturally. This animal model demonstrates biological clearance: binding-and-elimination evidence that plastics that would normally remain in tissue and in the gut are instead bound, passed through, and expelled by attaching to Qi601.
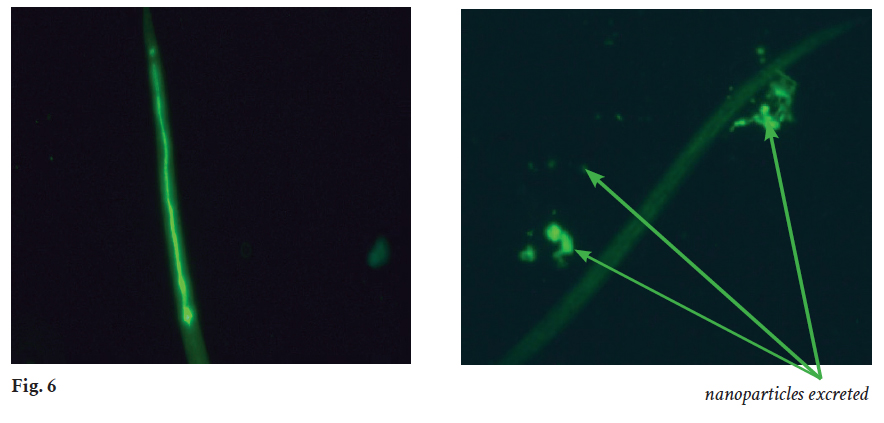
Fig. 6: In Vivo Evidence of Nanoplastic Clearance by Qi601 in C. elegans. Fluorescence microscopy of C. elegans nematodes fed bright green fluorescent nanoplastics shows intense intestinal fluorescence in controls (left panel) indicating epithelial accumulation. Worms co-fed Qi601 (bottom) display markedly reduced gut fluorescence, with bright green nanoparticles appearing in excreta (green arrows). The dull green coloring in the right panel is the result of autofluorescence of the C. elegans themselves. These results demonstrate that Qi601 binds the ingested bright green nanoplastics, preventing their adhesion to intestinal surfaces, and promotes natural elimination through the digestive tract.
How real is the performance of Qi601 under conditions that mimic real digestion in people? To answer this question, Quorum Innovations conducted studies using human simulated gastric fluids—an acidic pH with digestive enzymes under both fasted and fed states. Qi601 biofilm aggregates retained the activity and presumably retained their structural integrity by sequestering up to 99% of introduced nanoparticles in human digestive environment. The supernatants of the samples with and without Qi601 were measured by spectrophotometry. Once bound, plastic nanoparticles remained attached to Qi601 in the human gastric fluid environment, demonstrating a real-world, plausible pathway in which Qi601 binds plastics before they can reach or interact with the gut lining. This activity demonstrates the Qi601 can effectively bind the nanoplastic particles right from the beginning and from where the exposure to nanoplastics starts – in digestive fluid. Qi601 retains its binding capabilities even in stomach acid, holding onto the captured plastics so that they can safely pass out of the body.
Intriguingly, evidence from Quorum Innovations studies suggests that Qi601’s protective action may extend beyond interception at the epithelial surface. While its ability to bind nanoplastics in the gastric environment and promote natural excretion from the animal experiments is already demonstrated, new findings indicate that its influence may reach inside the cell itself. Nanoplastics can enter human epithelial cells through multiple pathways, such as passive diffusion across the cell wall and clathrin-mediated endocytosis — becoming trapped within intracellular vesicles such as endosomes and lysosomes. Quorum’s researchers are now exploring whether Qi601, by establishing a strong trans-epithelial binding gradient, creates a high-affinity extracellular sink that facilitates the efflux of internalized particles. In this model, the aggregates of Qi601 outside the cell could act as a molecular magnet, “pulling” nanoplastics back through the membrane or encouraging their release via natural vesicular recycling and exocytic export. Fluorescence microscopy and time-lapse imaging have shown declining intracellular fluorescence after Qi601 exposure, raising the remarkable possibility that this postbiotic biofilm shield can not only block new entry but also draw existing plastics out of human cells.
Bright-field and fluorescence microscopy images of human colonic epithelial cells suggest Qi601’s ability to remove nanoplastics that have already entered cells. In these experiments, cells were exposed to 100-nanometer fluorescent polystyrene nanoplastic spheres (green) for 24 hours without Qi601 treatment. Cells not treated with Qi601 show numerous bright puncta, representing aggregates of approximately 1,000 to 10,000 nanoplastic particles each, visible throughout the cytoplasm, confirming intracellular accumulation (Fig. 7, left upper and lower panels). In an identical parallel experiment, cells were first exposed to nanoplastics for 24 hours and then treated with Qi601 for an additional 4 hours. Following Qi601 treatment, intracellular fluorescence intensity markedly decreased, and nanoplastic aggregates appeared diminished or absent, suggesting active removal of internalized particles by Qi601 (Fig. 7,right upper and lower panels). These findings provide direct visual evidence that Qi601 not only prevents plastic entry into cells but suggests that it can also draw out and clear pre-existing intracellular nanoplastics—a previously unreported plastic decontamination mechanism in human epithelial models.

Figure 7: Qi601 Facilitates Removal of Intracellular Nanoplastics from Human Colon Cells. Bright-field and fluorescence microscopy of human colon cells (Caco2) show Qi601’s plastic decontamination effects. In untreated cells (left panels), 100-nanometer fluorescent nanoplastic particles (green spheres) accumulate throughout the cytoplasm, forming dense intracellular clusters. After 24 hours of exposure to nanoplastics followed by 4 hours of Qi601 treatment (right panels), intracellular fluorescence was markedly reduced, indicating that nanoplastics were drawn out and sequestered by Qi601 aggregates. These findings demonstrate that Qi601 not only prevents nanoplastic entry into epithelial cells but may also facilitate the removal of internalized particles — a novel, dual-action plastic decontamination mechanism.
Fluorescence microscopy of human colonic epithelial cells exposed to 100-nanometer fluorescent polystyrene (PS) nanoplastics for 24 hours reveals clear evidence of cellular uptake and intracellular accumulation. The upper left panel (Fig. 8) demonstrates the scale, appearance and distribution of the nanoplastics, appearing as a fine dusting of bright green fluorescent particles of the 100-nanometer nanospheres when not grouped together in endosomes. This is the typical appearance of 100-nanometer fluorescent plastics at this microscope resolution. Without Qi601 protection, punctate green fluorescent spheres are seen clustering along the nuclear and cellular boundaries within the cytoplasm, indicating that the nanoplastic particles are being actively internalized by the human colon cells (Fig. 8, top right panel). Higher-magnification (Fig. 8, bottom right panels) confirm that these particles are not merely adherent to the cell surface but likely localized within the cytoplasm and concentrated in the perinuclear region, suggesting entrapment within endosomal or lysosomal compartments.30 Together, these findings define the baseline state of nanoplastic interaction with intestinal epithelial cells, showing that in the absence of Qi601, nanoplastics can penetrate human colon cells and accumulate intracellularly. These intracellular spheres represent approximately 1000 nanoplastic particles concentrated in endosomes (Fig. 8, bottom right panel). This establishes the reference condition and control conditions for subsequent experiments.
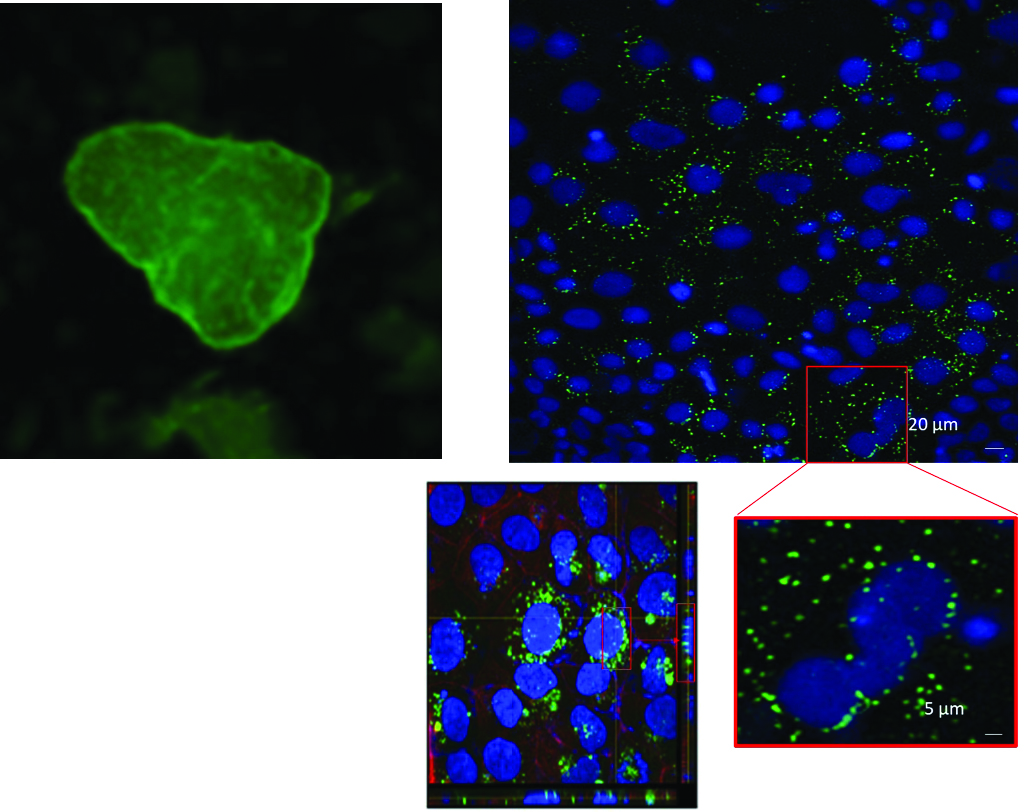
Figure 8: Nanoplastic Internalization in Human Colon Cells Without Qi601 Protection. Fluorescence microscopy of human colon cells exposed for 24 hours to 100-nanometer fluorescent polystyrene (PS) nanoplastic spheres (green) demonstrates active cellular uptake and intracellular accumulation. The upper left panel illustrates the appearance and scale with diffuse “dust-like” distribution of the 100-nanometer nanoplastics on Qi601. In stark contrast, the main composite images at the right upper and lower quadrants show bright punctate fluorescence within the cytoplasm and along nuclear boundaries, indicating internalization of thousands of nanoparticles grouped into spheres. Magnified views reveal nanoplastic localization in the perinuclear region and within the cytoplasm, suggesting uptake into endosomal or lysosomal compartments. These findings establish the baseline condition in which nanoplastics penetrate and persist inside human colon cells, forming dense intracellular clusters in the absence of Qi601 protection.
In this set of experiments at Quorum Innovations, fluorescence microscopy of human colonic epithelial cells demonstrates the plastic decontamination effects of Qi601 following exposure to fluorescent 100-nanometer nanoplastics. In the untreated control group (Fig. 9, left panels), cells were exposed for 24 hours to 100-nanometer fluorescent polystyrene nanoplastic spheres (green), with nuclei of the cells stained blue. The resulting images show dense green fluorescence throughout the cytoplasm, with aggregates concentrated near the nucleus, indicating perinuclear endosomal accumulation and active internalization of nanoplastic particles. The magnified view highlights the tight clustering of nanoparticles within intracellular compartments (Fig. 9, left lower panel). In contrast, the Qi601-treated group (Fig. 9, right upper and lower panels) exposed to the same nanoplastics for 24 hours and subsequently receiving Qi601 for 4 hours — exhibits a marked reduction in intracellular fluorescence. Instead, green fluorescent nanoplastic spherical particles have largely disappeared, with only remnants remaining intracellularly. (Fig. 9, right upper and lower panels). Any remaining fluorescent nanoparticles after treatment with Qi601, have been reduced to a fine dusting once they’ve been unbound and appear to be redistributed and faintly clustered along extracellular Qi601 aggregates (magenta), suggesting that Qi601 binds and extracts plastic particles from within cells toward the extracellular environment (Fig. 9, left panel). These findings provide direct visual evidence that Qi601 functions not only as a barrier that prevents nanoplastic entry but also as a plastic decontamination agent capable of facilitating the removal of internalized plastics from human epithelial cells. This suggests Qi601’s capacity to bind and extract plastic particles from the cellular environment, effectively lowering intracellular burden even after uptake has occurred. Together, these results provide visual confirmation that Qi601 acts both as a preventive barrier and a post-exposure plastic decontamination agent, removing nanoplastics from within human colon cells.
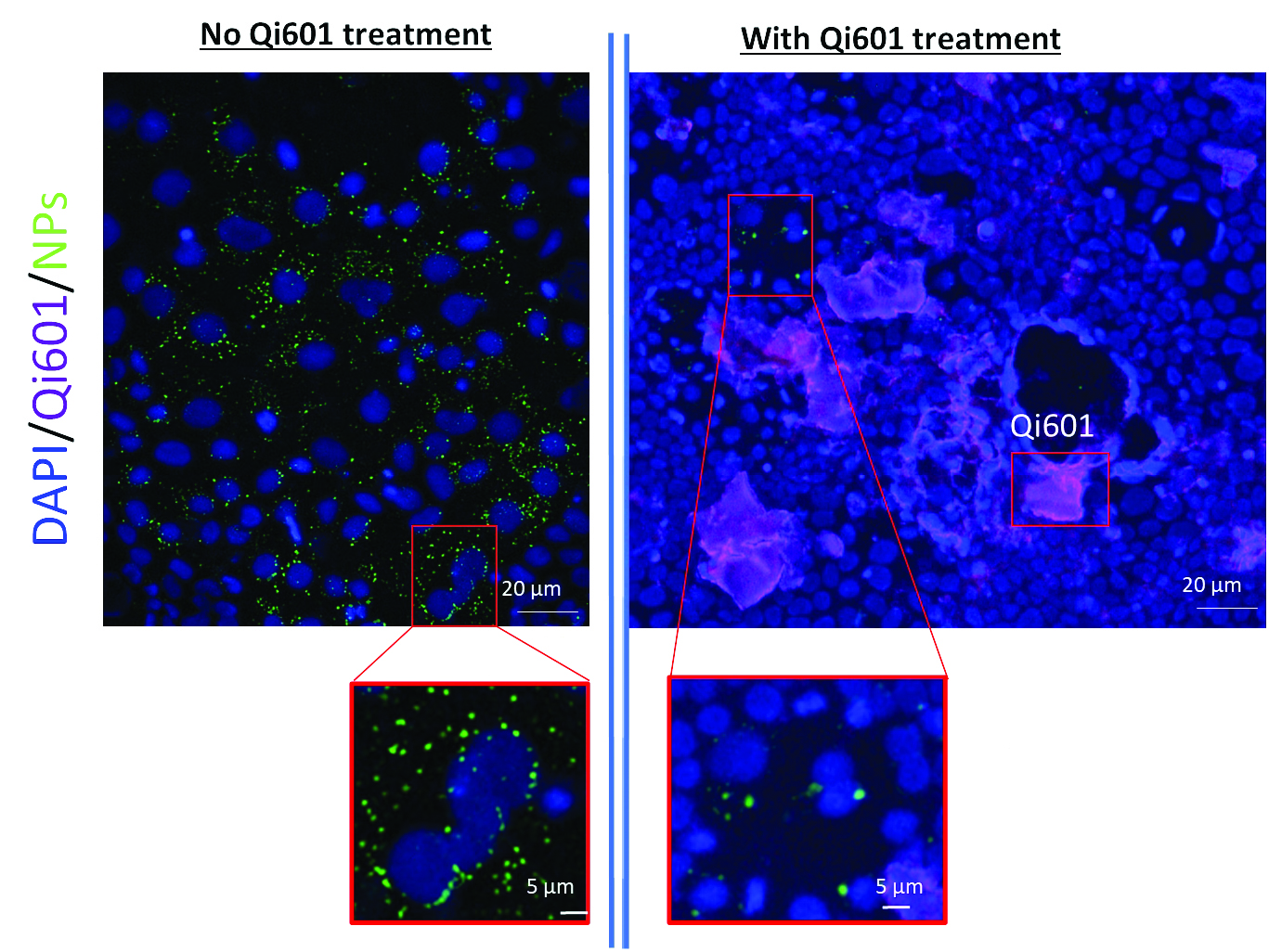
Figure 9: Qi601 Reduces Intracellular Nanoplastics in Human Colon Cells After 24 Hour Exposure. Fluorescence microscopy of human colonic epithelial cells demonstrates the plastic decontamination effects of Qi601 following nanoplastic exposure, In untreated controls (left panels), cells exposed for 24 hours to 100-nanometer fluorescent polystyrene nanoplastic spheres (green) show dense cytoplasmic and perinuclear accumulation, indicating active internalization via endosomal pathways. Magnified views in the bottom left highlight tight clustering of nanoparticles near the nucleus. In contrast, Qi601-treated cells (right panels)—after identical 24-hour nanoplastic exposure followed by 4 hours of Qi601 treatment—display markedly reduced intracellular fluorescence. Most nanoplastic particles have been cleared or redistributed to extracellular Qi601 aggregates (magenta), leaving only faint residual signals or a fine particulate “dusting.” These findings indicate that Qi601may function as a post-exposure plastic decontamination agent, actively extracting internalized nanoplastics from human colon cells and reducing intracellular burden.
Fluorescence microscopy and quantitative analysis suggest that Qi601 facilitates the removal of intracellular nanoplastics (NPs) from human colon epithelial cells through a mechanism likely driven by a transepithelial barrier gradient. In untreated conditions, cells internalize fluorescent nanoplastic particles, which persist within the cytoplasm and perinuclear regions, maintaining a steady intracellular burden. However, upon addition of Qi601 following exposure, intracellular fluorescence intensity decreases significantly, consistent with the hypothesis that extracellular Qi601 aggregates act as an extracellular high-affinity sink. This creates a binding gradient that favors the outward movement of nanoplastics—possibly through vesicular recycling, endosomal export, or passive efflux—toward the extracellular Qi601 biofilm surface where nanoplastics are bound, sequestered and prevented from reentry. This gradient-driven clearance mechanism may mirror the same physical and electrostatic binding principles that enable Qi601 to prevent plastic attachment in the first place, thus uniting prevention and plastic decontamination within a single mechanistic framework. These results indicate that Qi601 not only intercepts nanoplastics before they enter cells but can also reverse intracellular accumulation transforming the epithelial interface from a passive barrier into an active plastic decontamination system. In this model, extracellular biofilm Qi601 aggregates act as high-affinity sinks, creating a concentration gradient that promotes outward transport of intracellular NPs through vesicular recycling, endosomal export, or passive efflux. These findings reinforce microscopy observations, suggesting that Qi601 not only prevents plastic entry into cells but may also reverse intracellular accumulation —transforming the epithelial interface from a passive barrier into an active plastic decontamination system.
Quantitative data reinforces what the microscopy images reveal: Qi601 markedly reduces intracellular nanoplastic burden following exposure (Fig. 10). Measurements of intracellular fluorescence show a significant decline in internalized nanoplastics particles after Qi601 is introduced, mirroring the visual reduction in cytoplasmic and perinuclear nanoplastic clusters. This numerical confirmation strengthens the interpretation that Qi601’s external high-affinity binding surface creates a transepithelial gradient capable of drawing particles outward, preventing their re-entry, and reducing their intracellular persistence. Together, the quantitative analyses and visual evidence converge on the same conclusion — that Qi601 may actively facilitate its clearance once inside the cell.
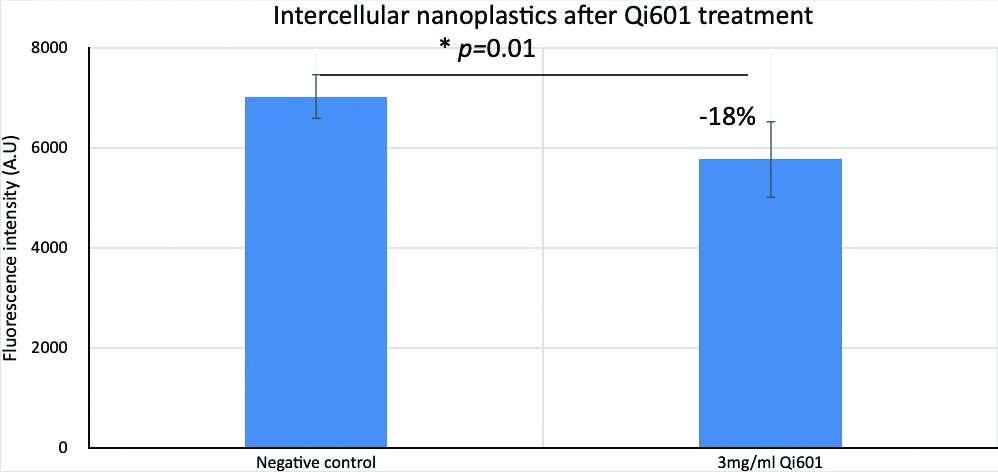
Figure 10: Quantitative fluorescence analysis of human colon epithelial cells demonstrates that treatment with Qi601 significantly decreases intracellular nanoplastic (NP) accumulation. Cells exposed to 100-nanometer fluorescent polystyrene nanoplastics for 24 hours were subsequently treated with 3 mg/mL Qi601 exhibited an 18% reduction in intracellular fluorescence intensity compared to untreated controls (p = 0.01). This statistically significant decrease supports the hypothesis that Qi601 facilitates removal of internalized nanoplastics via a transepithelial barrier gradient mechanism.
This convergence of barrier protection and intracellular clearance demonstrates how microbiology-inspired design can be translated into tangible human protection — where the body is no longer merely a passive recipient of environmental contaminants but can engage mechanisms for active participant in their removal. Qi601 biofilm aggregates are not live cells yet are bio-interactive, forming a surface that prevents micro- and nanoplastics from adhering to or penetrating cells while also facilitating their removal once internalized. Does Qi601 work by lowering the concentration of free particles at the outside cell surface and provide a high-affinity sink in close proximity to the cellular surface, so the aggregates may favor decreasing the intracellular concentration? This mechanism would mark a paradigm shift in the biological handling of synthetic pollutants. By coupling these innate transport routes with an external, high-affinity sequestration platform, Qi601 transforms the epithelial barrier from a passive boundary into an active plastic decontamination interface. These findings mark a significant step forward in translating microbiology-inspired design into tangible human protection.
Quorum Innovations researchers are testing whether Qi601 is functioning as a strong trans-epithelial binding gradient to facilitate active transport out of the cell and can “pull” intracellular nanoplastics back out of human cells. Alternatively, or in addition, Qi601 decreases intracellular nanoplastic particles by inducing recycling or exocytic export from endosomal compartments toward the outside of the cell, to the lumen, where particles immediately bind and are sequestered. In both cases, this “active export” supported by experimental findings remains an evolving hypothesis that Quorum Innovations is validating with live-cell trafficking, endosomal markers, and inhibitor studies, early time-lapse imaging and declining intracellular fluorescence after exposure with confocal microscopy. This activity represents a complete paradigm shift —showing that intracellular plastic burden might be reversible, and that Qi601 can act as both a shield that prevents binding to human tissues and as a technology that can remove intracellular particles such as nanoplastics.
Taken together—fluorescent cell imaging, AFM topology, test tube nanoplastics binding assays, gastric stability with near-complete nanoparticle binding, and nematode clearance—these data portray postbiotic biofilm technology in which aggregates sequester micro- and nanoplastics from reaching the epithelium and may help reverse intracellular plastic burdens by coupling intracellular trafficking to an external, high-affinity sink.
Quorum Innovations researchers are testing whether Qi601 is functioning as a strong trans-epithelial binding gradient to facilitate active transport out of the cell and can “pull” intracellular nanoplastics back out of human cells. Alternatively, or in addition, Qi601 decreases intracellular nanoplastic particles by inducing recycling or exocytic export from endosomal compartments toward the outside of the cell, to the lumen, where particles immediately bind and are sequestered. In both cases, this “active export” supported by experimental findings remains an evolving hypothesis that Quorum Innovations is validating with live-cell trafficking, endosomal markers, and inhibitor studies, early time-lapse imaging and declining intracellular fluorescence after exposure with confocal microscopy. This activity represents a complete paradigm shift —showing that intracellular plastic burden might be reversible, and that Qi601 can act as both a shield that prevents binding to human tissues and as a technology that can remove intracellular particles such as nanoplastics.
Taken together—fluorescent cell imaging, AFM topology, test tube nanoplastics binding assays, gastric stability with near-complete nanoparticle binding, and nematode clearance—these data portray postbiotic biofilm technology in which aggregates sequester micro- and nanoplastics from reaching the epithelium and may help reverse intracellular plastic burdens by coupling intracellular trafficking to an external, high-affinity sink.
Quorum Innovations: Developing a Broader Biofilm Shielding Strategy
Qi601 as a defense against absorption of plastics is not the only vision for Quorum Innovations development pipeline. Building on the success of the Biofilm Shield™, Quorum is also exploring adjuvant strategies designed to complement Qi601’s gut sequestration. These include engineered dietary fibers that can further bind micro- and nanoplastics, products aimed at intercepting aerosolized plastics before they embed in lung tissue, and surface treatments to capture plastics in food and water prior to ingestion. In parallel, Quorum Innovations is also developing diagnostic platforms to measure an individual’s progress — tracking plastic reduction in the body — as well as tools to assess and monitor plastic exposure within home environments. Together, these efforts represent a broader protective ecosystem that extends the Qi601 biofilm shield from the gut to the air we breathe, the food we eat, and the spaces we live in. Yet Qi601 stands apart as one of the first internal defenses against plastics with visible, quantitative proof of efficacy. Together, these efforts represent an evolving protective ecosystem that extends the Qi601 Biofilm Shield™ from the gut to the lungs, the skin, and even the domestic environment — creating a continuous, multilayered defense that mirrors the natural microbiome’s role in protecting human surfaces.
At the core of these innovations lies the same fundamental biological principle demonstrated by Qi601: the transepithelial barrier gradient. This mechanism, which enables the removal of internalized nanoplastics by creating a high-affinity extracellular sink, has the potential to be generalized beyond plastics. The same surface-driven forces that draw nanoplastics out of epithelial cells may also apply to other intracellular particles — heavy metals, persistent organic pollutants, or inflammatory microparticles — that similarly accumulate within human tissues. By coupling natural biofilm surface properties with engineered binding specificity, Quorum aims to develop a next generation of microbiome-inspired barrier technologies that can neutralize contaminants already within the body. In this way, the transepithelial barrier gradient represents more than a mechanism — it is a new platform for plastic decontamination, offering a practical, biologically grounded, safe solution to the rising tide of environmental exposures that nearly everyone can agree simply do not belong inside the human body and are not going away anytime soon
Taken together—fluorescent cell imaging, AFM topology, test tube nanoplastics binding assays, gastric stability with near-complete nanoparticle binding, and nematode clearance—these data portray postbiotic biofilm technology in which aggregates sequester micro- and nanoplastics from reaching the epithelium and may help reverse intracellular plastic burdens by coupling intracellular trafficking to an external, high-affinity sink.
The arc of Qi601’s story is striking. Quorum Innovations technology has evolved from a biological defense to defend soldiers against battlefield chemicals to a technology to protect families at the dinner table. In the age of the Plasticene — where plastics lace the soil and saturate our food and air — Qi601 offers a new kind of shield. It is not a cure, nor a replacement for environmental reform, but it is a defense we can use now. The roots of this innovation stretch back even further. Quorum Innovations began with a foundational insight into the human microbiome – that it is not merely a collection of microbes but a protective system, with biofilms representing a special phenotype evolved to guard human surfaces against invasion, be it biological or environmental. This concept of “microbial barrier protection” guided Quorum’s earliest research and was later advanced through collaborations with the Department of Defense and DARPA, which recognized the potential of using safe bacteria and biofilm-derived technologies to shield warfighters against chemical and biological threats.
Qi601 embodies that lineage. Built from natural biofilm microbial aggregates, it carries forward the idea that nature’s own barriers can be repurposed to meet the new challenges of the modern world. Qi601 embodies the definition of a Probiomic® — a biologically inspired, probiotic-derived technology from biofilm microbial aggregates. Built from Limosilactobacillus fermentum, an FDA-reviewed and GRAS-certified organism, the Qi601 Probiomic® represents a new class of microbiome-based innovation that captures the protective architecture of nature’s own biofilms to create safe, functional barriers for human health. It is not alive, but it behaves with biological intelligence — a reengineered extension of the microbiome’s innate defense system, designed to protect the body from modern environmental challenges such as micro- and nanoplastics. In this way, Qi601 represents one of the first, if not the first, conceptual advances capable of both defending against and potentially helping to remove the plastics that have already been absorbed into our bodies.
What makes this story profoundly elegant is that Quorum did not invent a new chemistry— like many inventions, it looked back to nature for inspiration. QI founders went back to the source — the human microbiome — and harnessed its inherent protective architecture, refining it into a tangible technology that now serves as a new class of biological defense. Quorum founders simply rediscovered what evolution had already perfected: for millions of years, bacteria have shielded human tissues from environmental insults. Through this lens, Qi601 is a translation of evolutionary wisdom into modern remedies. By replicating the natural surface dynamics of protective biofilms, Qi601 establishes a transepithelial barrier gradient that results in both preventing the uptake of nanoplastics and promoting their removal once inside cells. This same mechanism may extend far beyond plastics, offering potential defense and plastic decontamination from other intracellular particles such as heavy metals, pesticide residues, and industrial pollutants — all substances that, like nanoplastics, simply do not belong in the human body. What began as a DARPA-inspired innovation to protect soldiers from chemical and biological warfare has evolved into a technology now protecting families at their dinner tables — a continuation of nature’s own design, reengineered to meet the toxic realities of the 21st century.
